Pi Network Orbital Diagram
Free Printable Pi Network Orbital Diagram
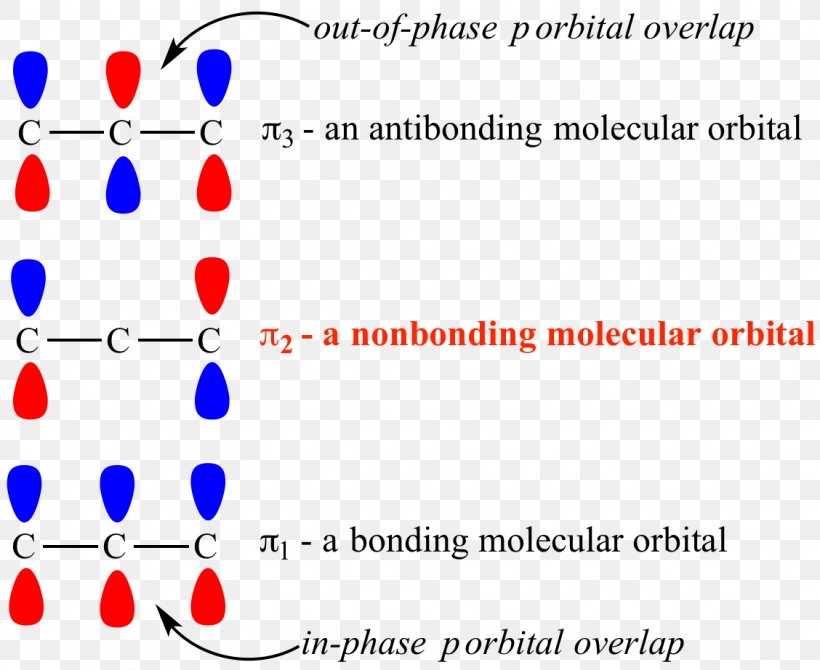
Will any of the orbitals be non bonding orbitals.
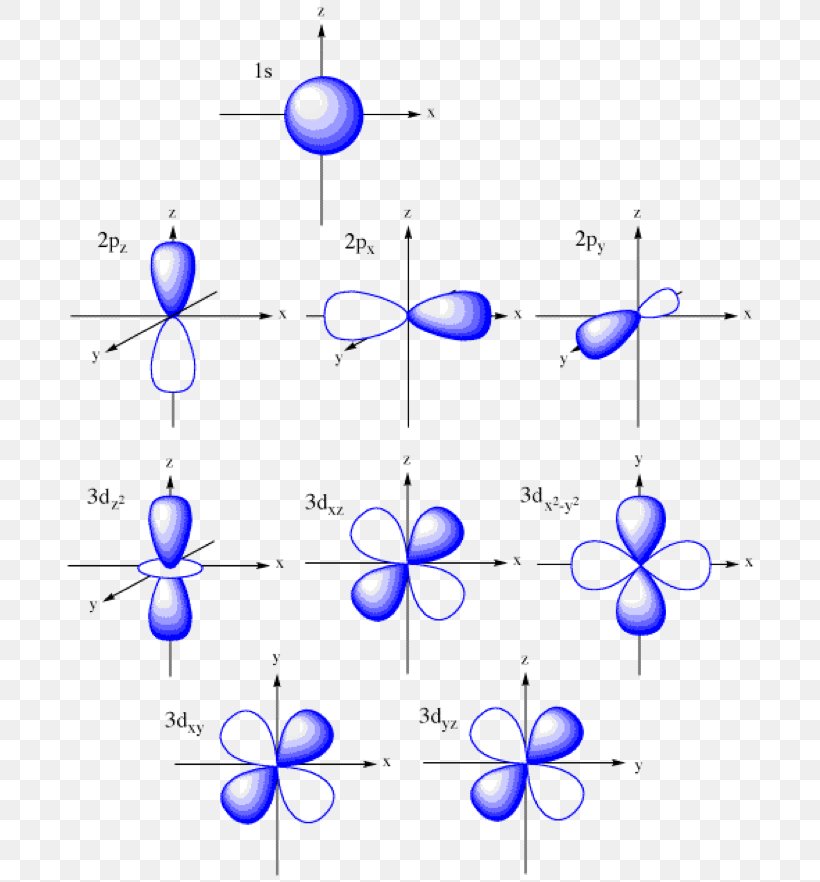
Pi network orbital diagram. Combine each h 1s orbital with a c 2sp 2 orbital to make a sigma bonding and a sigma antibonding. The atomic number of nitrogen is 7. The electronic configuration of n2 is kk ?? 2s 2 ?? 2s 2 ?? 2p x 2 ?? 2p y 2 ?? 2p z 2. S orbitals are involved in chemical bonding.
N b 8 na 2. Bonding molecular orbital the bonding electron density lies above and below or in front and in back of the bonding axis with no electron directly on the bonding axis since 2p orbitals do not have any electron density at the nucleus. For a limited time you can join the beta to earn pi and help grow the network. The orbitals responsible for bonding are not shown.
The molecular orbital energy level diagram of h 2 molecule is given in fig. As with borane make 2sp 2 hybrid orbitals on each carbon from the 2s 2p x and 2p y atomic orbitals. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. Construct and fully label the pi molecular orbital diagram for 1 3 butadiene.
And which is the lumo. Pi is a new digital currency being developed by a group of stanford phds. Below are partial mo diagrams for metal ligand bonding in oh. 4 lecture 2 pi bond ??.
The energy scale is expanded relative to the complex mo diagram. Bond order n b n a 2 2 0 2 2 i. Each carbon forms 3 sigma bonds and has no lone pairs. A 2p z orbital remains on each carbon.
Ethene sp2 hybridization with a pi bond. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. The molecular orbital electronic configuration of hydrogen molecule is s 1s 2. Determine which diagram is for the donor and acceptor cases.
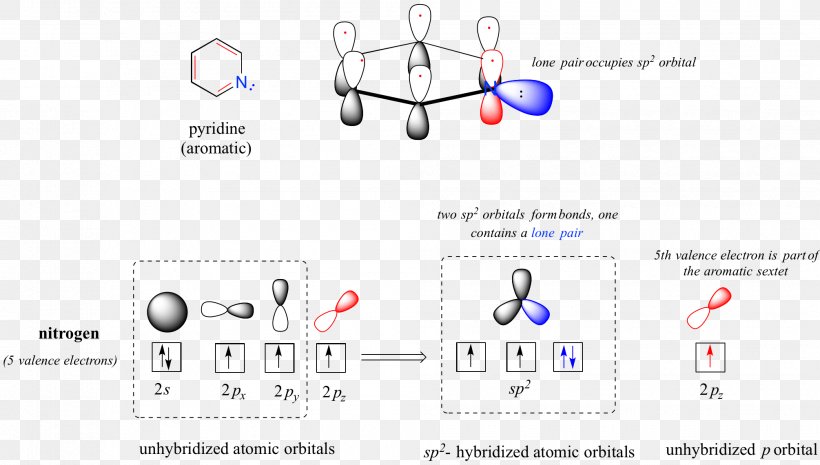
The bond order of h 2 molecule can be calculated as follows. Solution for draw a diagram to show the orbitals involved in forming the conjugated six pi electron system present in aromatic heterocycle such as pyridine. The number of p orbitals in the system. They can take part in the formation of sigma bonds but these s orbitals cannot form pi bonds the spherical shape tells us the most probable region where the electrons can be found.
Which molecular orbital is the homo. The lewis structure of the molecule ch 2 ch 2 is below. Here n b 2 and n a 0. Bond order value of 3 means that n 2.
Label the mos with the appropriate symmetry labels and the o. The double bond in c 2 consist of both pi bonds because the four electrons are present in the two pi molecular orbitals.